 Urgent update on SMA treatment in Ukraine, May 2024
20 Травня, 2024
Акції, Журналістам, Закон
Urgent update on SMA treatment in Ukraine, May 2024
20 Травня, 2024
Акції, Журналістам, Закон
Dear Colleagues, Friends, and Interested Parties around the World,
This message provides a brief overview of the current situation regarding Spinal Muscular Atrophy (SMA) treatment in Ukraine, particularly within the context of broader initiatives for orphan diseases in the National Policy.
Despite the ongoing full frame russian invasion, the Ukrainian government (Ministry of Health) is continuing efforts to implement previously established measures regarding orphan diseases in context of National Plan.
However, there is huge concern by patient’s community in the assessment of real situation (results) in comparison to the official statements of the Ministry of Health and other relevant structures, representatives of the authorities in this matter.
We ask any involved persons (official strutures, patient organizations) of EU take into account this message, support SMA community and do any proper acts to improve situation. Any requests for additional information has to be addressed to the Foundation “Children with SMA” or here.
Report as on May 2024.
Despite the approval of the Concept for the Development of the System of Medical Care for Patients with Rare (Orphan) Diseases for 2021-2026 [1], implementation of the plan is not systematic and is formal now. The attitude towards SMA treatment is indicative of all orphan diseases. Common challenges as well as for SMA are
- absence of Patient registry
- Access to EMA-approved disease modifying therapies (DMTs)
- Standards of care (SoC) & treatment guidelines
- HCP consultations reimbursement
From end of 2021 to 2023, a legislative initiative was adopted in Ukraine that allowed pharmaceutical companies to enter into agreements with the State for patients’ access to innovative therapies through managed access agreements. However, only 2 innovative molecules were accepted through this mechanism [2] during all time. One of these molecules is risdiplam – a drug for spinal muscular atrophy (SMA). Although another SMA molecule, nusinersen, is registered in Ukraine, it has been ignored by the Ministry of Health for access to it so far. Adults living with SMA are unfortunately not eligible for the reimbursement of nusinersen. Regarding risdiplam, while initially no adults living with SMA were eligible for reimbursement, criteria have been recently widened to adults living with SMA of any age, but only for type 1. In the same time patients with type 3, based only on SMN2 copies prognozis, without symptoms, detected by national newborn screening, do not have access to any treatment.
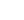
Short report by SMA Europe [3] complements the benchmarking report on SMA adult care published in 2024 to assess how care is provided for adults with spinal muscular atrophy (SMA) living in 22 European countries [4] , as well as the OdySMA project, an SMA access atlas [5]. The objective of this report is to provide an evidence-based resource to engage EU and national policymakers and increase awareness about the care challenges faced by adults living with SMA and on our point of view other SMA categories. It also features calls-to-action for policy modifications at both national and EU level.
Adults living with SMA as well as other categories of SMA patients have very limited national access to disease modifying treatments available across Europe. Although a registry from the Ukrainian Foundation for Children with SMA currently exists that collects data on patients living with SMA, it is not being used by the Ministry of Health. There is insufficient reimbursement for both medical and non-medical assistive devices, along with a general lack of support to enable adults living with SMA to live independently.
SMA Europe, with the endorsement of the Ukrainian Foundation for Children with SMA, calls on policymakers, healthcare systems, and the medical community:
to take coordinated action, in collaboration with the SMA patient community, to address current inequalities in access to pharmacological treatment, which would include eliminating any existing barriers restricting access to available disease modifying therapies, such as restricting eligibility for treatment to certain SMA types or ages;
to be ensure the network of SMA/NMD treatment centers facilitates SMA training programs for HCPs, including physiotherapists, to ensure multidisciplinary care teams within those, and HCPs outside of those, deliver care on the basis of best practices.
- https://zakon.rada.gov.ua/laws/show/377-2021-%D1%80#Text
- Transcript of the meeting of the Verkhovna Rada Committee, p. 42, DMYTRIEVA O.O. https://komzdrav.rada.gov.ua/uploads/documents/33444.pdf
- https://odysma-dev-cms.gpm.digital/uploads/M_XX_00016273_Ukraine_SMA_Europe_Policy_report_Feb_2024_3678809ec3.pdf
- https://odysma-dev-cms.gpm.digital/uploads/0222_SMA_Main_Report_290224_V15_7ee5341b81.pdf
- https://odysma.sma-europe.eu/countries/ukraine